
Sarah Stewart Johnson was a college sophomore when she first stood atop Hawaii’s Mauna Kea volcano. Its dried lava surface was so different from the eroded, tree-draped mountains of her home state of Kentucky. Johnson wandered away from the other young researchers she was with and toward a distant ridge of the 13,800-foot summit. Looking down, she turned over a rock with the toe of her boot. To her surprise, a tiny fern lived underneath it, having sprouted from ash and cinder cones. “It felt like it stood for all of us, huddled under that rock, existing against the odds,” Johnson says.
Her true epiphany, though, wasn’t about the hardiness of life on Earth or the hardships of being human: It was about aliens. Even if a landscape seemed strange and harsh from a human perspective, other kinds of life might find it quite comfortable. The thought opened up the cosmic real estate, and the variety of life, she imagined might be beyond Earth’s atmosphere. “It was on that trip that the idea of looking for life in the universe began to make sense to me,” Johnson says.
Later, Johnson became a professional at looking. As an astronomy postdoc at Harvard University in the late 2000s and early 2010s she investigated how astronomers might use genetic sequencing—detecting and identifying DNA and RNA—to find evidence of aliens. Johnson found the work exciting (the future alien genome project!), but it also made her wonder: What if extraterrestrial life didn’t have DNA or RNA or other nucleic acids? What if their cells got instructions in some other biochemical way?
As an outlet for heretical thoughts like this, Johnson started writing in a style too lyrical and philosophical for scientific journals. Her typed musings would later turn into the 2020 popular science book The Sirens of Mars. Inside its pages, she probed the idea that other planets were truly other, and so their inhabitants might be very different, at a fundamental and chemical level, from anything on this world. “Even places that seem familiar—like Mars, a place that we think we know intimately—can completely throw us for a loop,” she says. “What if that’s the case for life?”
If Johnson’s musings are correct, the current focus of the hunt for aliens—searching for life as we know it—might not work for finding biology in the beyond. “There’s this old maxim that if you lose your keys at night, the first place you look is under the lamppost,” says Johnson, who is now an associate professor at Georgetown University. If you want to find life, look first at the only way you know life can exist: in places kind of like Earth, with chemistry kind of like Earthlings’.
Much of astrobiology research involves searching for chemical “biosignatures”—molecules or combinations of molecules that could indicate the presence of life. But because scientists can’t reliably say that ET life should look, chemically, like Earth life, seeking those signatures could mean we miss beings that might be staring us in the face. “How do we move beyond that?” Johnson asks. “How do we contend with the truly alien?” Scientific methods, she thought, should be more open to varieties of life based on varied biochemistry: life as we don’t know it. Or, in a new term coined here, “LAWDKI.”
Now Johnson is getting a chance to figure out how, exactly, to contend with that unknown kind of life, as the principal investigator of a new NASA-funded initiative called the Laboratory for Agnostic Biosignatures (LAB). LAB’s research doesn’t count on ET having specific biochemistry at all, so it doesn’t look for specific biosignatures. LAB aims to find more fundamental markers of biology, such as evidence of complexity—intricately arranged molecules that are unlikely to assemble themselves without some kind of biological forcing—and disequilibrium, such as unexpected concentrations of molecules on other planets or moons. These are proxies for life as no one knows it.
Maybe someday, if LAB has its way, they will become more than proxies. These signals could help answer one of humankind’s oldest questions—Are we alone?—and show us that we’re not so special, and neither is our makeup.
Life, Astro Life or Lyfe
Part of the difficulty in searching for life of any sort is that scientists don’t agree on how life started in the first place—or what life even is. One good attempt at a definition came in 2011 from geneticist Edward Trifonov, who collated more than 100 interpretations of the word “life” and distilled them into one overarching idea: it’s “self-reproduction with variations.” NASA formulated a similar working definition years earlier, in the mid-1990s, and still uses it to design astrobiology studies. Life, according to this formulation, “is a self-sustaining chemical system capable of Darwinian evolution.”
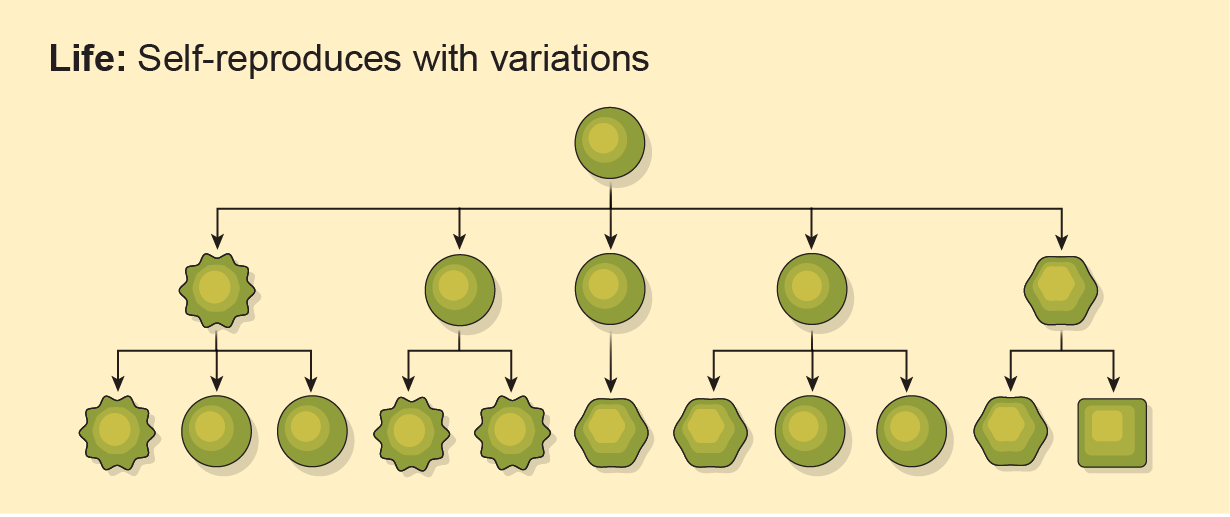
Neither of those classical definitions requires a particular chemistry. On Earth, of course, life runs on DNA: deoxyribonucleic acid. DNA is made up of two twisted strands, each comprising alternating sugar and phosphate groups. Stuck to every sugar is a base—the As (adenine), Gs (guanine), Cs (cytosine), and Ts (thymine). Together the bases and sugar-phosphates form nucleotides; DNA itself is a nucleic acid. RNA is kind of like single-stranded DNA—among other things, it helps translate DNA’s instructions into actual protein production.
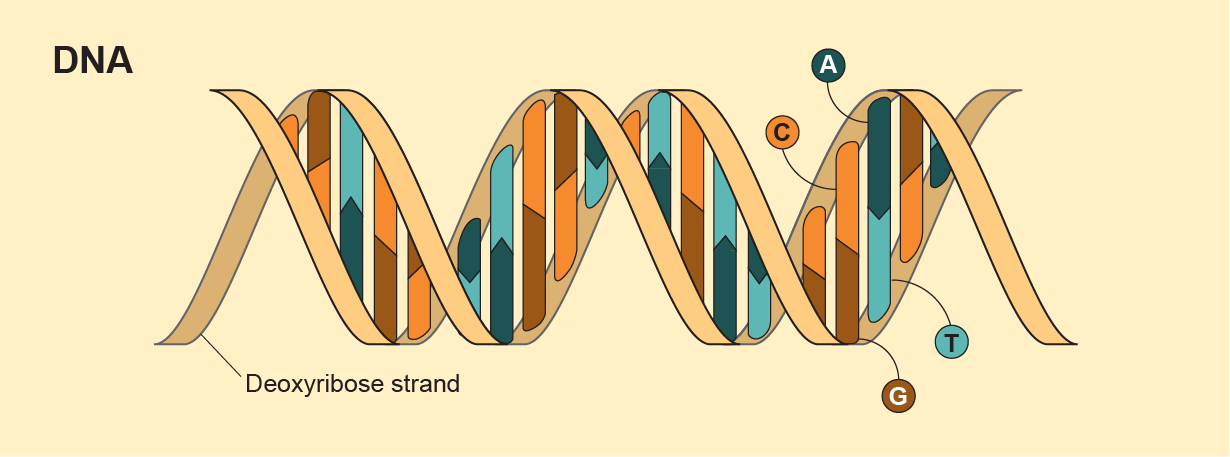
The simple letters in a genetic sequence, strung together in a laddered order, carry all the information needed to make you, squirrels and sea anemones. DNA can replicate, and DNA from different organisms (when they really, really love one another) can mix and meld to form a new organism that can replicate itself in turn. If biology elsewhere relied on this same chemistry, it would be life as we know it.
Scientists assume all forms of life would need some way to pass down biological instructions whose shifts could also help the species evolve over time. But it’s conceivable that aliens might not make these instructions out of the same chemicals as ours—or in the same shape. For instance, starting in the 1990s, Northwestern University researchers made SNAs, spherical nucleic acids.
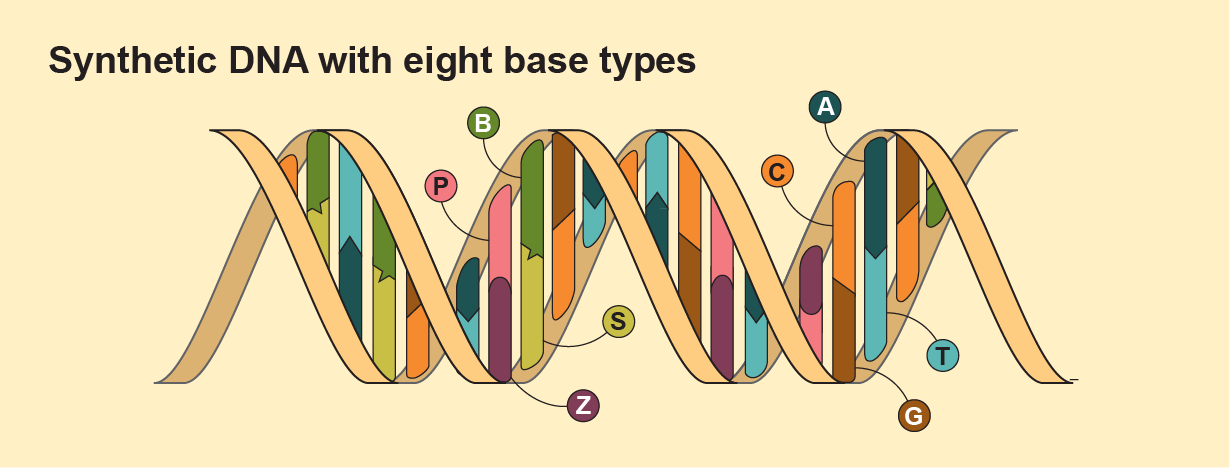
Alien life could have genetic code with, say, different bases. NASA-supported 2019 research, from the Foundation for Applied Molecular Evolution, successfully created synthetic DNA that used the four old-school bases and four new ones: P, Z, B and S. Scientists have also altered the strand part of genetic code, creating XNA—where X means anything goes—that uses a molecule such as cyclohexene (CeNA) or glycol (GNA), rather than deoxyribose. Big thinkers have long suggested that rather than using carbon as a base, as all these molecules do, perhaps alien life might use the functionally similar element silicon—meaning it wouldn’t have nucleic acids at all but other molecules that perhaps play the same role. If we can whip up such diversity in our minds and our labs, shouldn’t the universe be even more creative and capable?
It’s for that reason that LAB collaborator Leroy Cronin of the University of Glasgow doesn’t think scientists should even be talking about biology off-Earth at all. “Biology is unique,” he proclaims. RNA, DNA, proteins, typical amino acids? “Only going to be found on Earth.” He thinks someday people will instead say, “We’re looking for “astro life.” (LAWDKI has yet to catch on.)
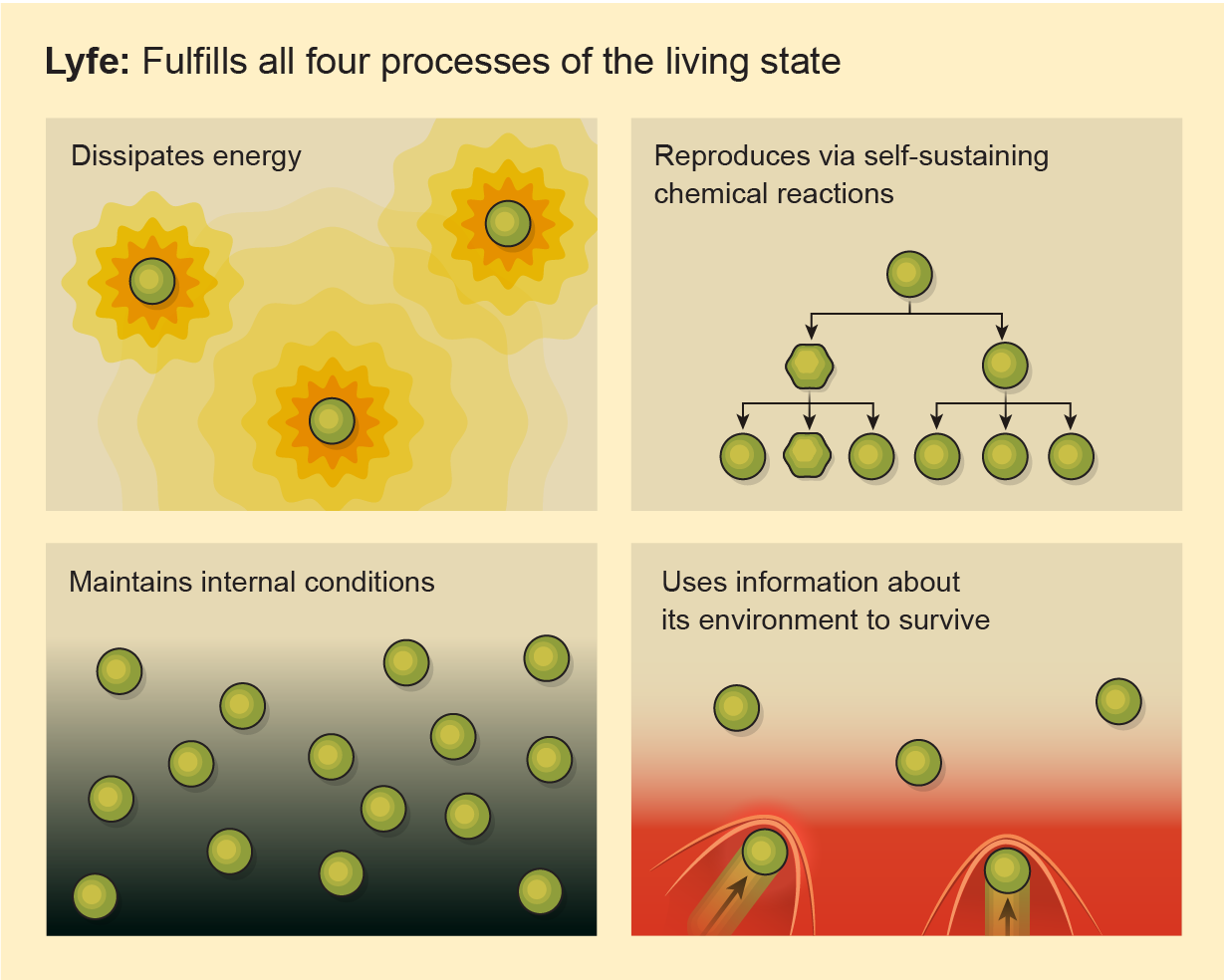
Stuart Bartlett, a researcher at the California Institute of Technology and unaffiliated with LAB, agrees with the linguistic critique. The search for weird life isn’t actually a search for life, Bartlett argues. It’s a search for “lyfe,” a term proposed in a 2020 article he co-authored in, ironically, the journal Life. “Lyfe,” the paper says, “is defined as any system that fulfills all four processes of the living state.” That means that it dissipates energy (by, say, eating and digesting), uses self-sustaining chemical reactions to make exponentially more of itself, maintains its internal conditions as external conditions change, and takes in information about the environment that it then uses to survive. “Life,” meanwhile, the paper continues, “is defined as the instance of lyfe that we are familiar with on Earth.”
Bartlett’s work, though separate from LAB’s, emerges from the same fascination: “That mysterious, opaque transition between things like physics and chemistry that we understand fairly well,” he says, “and then biology that is still shrouded in mystery.” How life becomes life at all is perhaps the most central question of astrobiology.
Trying to figure out how biology emerged on the planet we know best is the province of “origin of life” studies. There are two main hypotheses for how clumps of chemistry became lumps of biology—a process called abiogenesis. One holds that RNA arose able to make more of itself, because that’s what it does, and that it could also catalyze other chemical reactions. Over time that replication led to beings whose makeup relied on that genetic code. The “metabolism-first” framework, on the other hand, posits that chemical reactions organized in a self-sustaining way. Those compound communities and their chemical reactions grew more complex and eventually spit out genetic code.
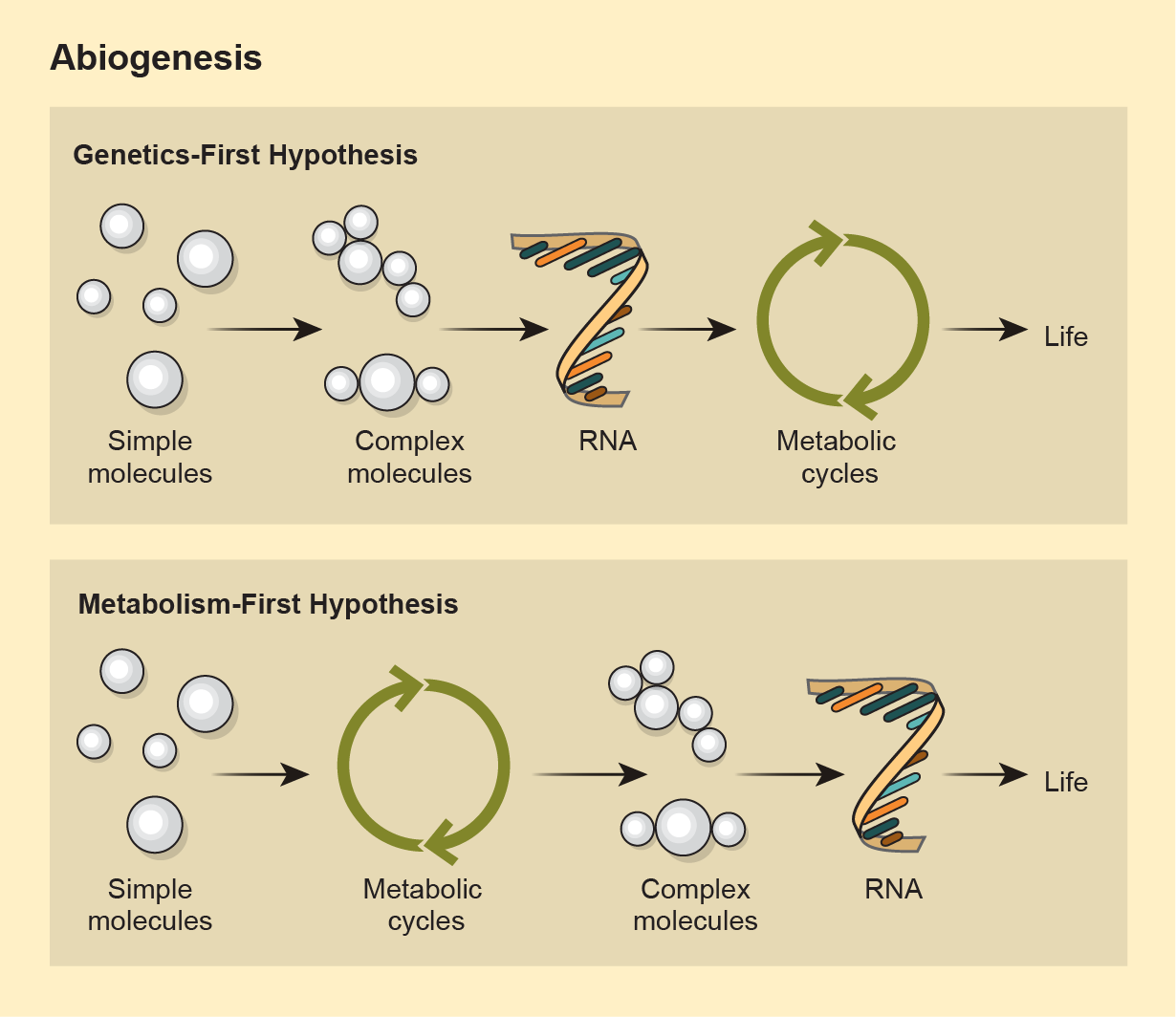
Those two main hypotheses aren’t mutually exclusive. John Sutherland, a chemist at the Medical Research Council Laboratory of Molecular Biology, is co-director of a group called the Simons Collaboration on the Origins of Life, which merges previous ideas about how one or another subsystem, such as genetics or early metabolism, came first. But if he’s being real, Sutherland admits he doesn’t understand how biology got started. No one does.
And until scientists know more about how things probably went down on the early Earth, Sutherland argues, there’s no way to estimate how common extraterrestrial anything might be. It doesn’t matter that there are trillions of stars in billions of galaxies: If the events that led to life are supremely uncommon, those many solar systems might still not be enough, statistically, to have resulted in abiogenesis—in other beings.
Bio-Agnostic
The first issue of the academic journal Astrobiology, more than two decades ago, featured an article by Kenneth Nealson and Pamela Conrad called “A Non-Earth-centric Approach to Life Detection.” But taking a non-Earth-centric approach isn’t easy for our brains, which formed in this environment. We are notoriously bad at picturing the unfamiliar. “It’s one of the biggest challenges we have, like imagining a color we’ve never seen,” Johnson says.
So astrobiologists often end up looking for aliens that resemble Earth life. Astronomers like to consider oxygen in an exoplanet atmosphere as a potential indicator of life—because we breathe it—although a planet can fill up with that gas in less lively ways. On Mars, researchers have been psyched by puffs of methane, organic molecules, and the release of gas after soil was fed a solution of what we on Earth call nutrients, perhaps indicating metabolism. They create terms like “the Goldilocks zone” for the regions around stars where planets could host liquid water, implying that what’s just right for Earth life is also just right everywhere else.
Even when scientists do discover biology unfamiliar to them, they tend to relate it to something familiar. For instance, when Antonie van Leeuwenhoek saw single-celled organisms through his microscope’s compound lens in the 17th century, he dubbed them “animalcules,” or little animals, which they are not.
Heather Graham, who works at NASA’s Goddard Space Flight Center and is LAB’s deputy principal investigator, sees van Leeuwenhoek’s discovery as a successful search for LAWDKI, close to home. The same description applies to scientists’ discovery of Archaea, a domain of ancient single-celled organisms first recognized in the 1970s. “If you reframe those discoveries as agnostic biosignatures in action, you realize that people have been doing this for a while,” Graham says.
Around 2016, Johnson joined their ranks, finding some like-minded nonbelievers who wanted to probe that darkness. At an invitation-only NASA workshop about biosignatures, Johnson sat at a table with scientists like Graham, gaming out how they might use complexity as a proxy for biology. On an exaggerated macroscale, the idea is that if you come across a fleet of 747s on Mars, you might not know where they came from, but you know they’re unlikely to be random. Someone, or something, created them.
After the meeting, Johnson and her co-conspirators put in a last-minute proposal to develop an instrument for NASA. It would find and measure molecules whose shapes fit physically together like lock and key because that rarely happens in random collections of chemical compounds but pops up all over living cells. The instrument idea, though, didn’t make the cut. “That’s when we realized, ‘Okay, we need to roll this back and do a lot more fundamental work,’” Graham says.
The space agency would give them a chance to do so, soon putting out a call for “Interdisciplinary Consortia for Astrobiology Research.” It promised multiple years of funding to dig deeper into Johnson and her associates’ lunch-table ideas. They needed a larger team, though, so they pinged planetary scientists, biologists, chemists, computer scientists, mathematicians and engineers—some space-centric to the core and others, Johnson says, “just beginning to consider the astrobiology implications of their work.” It was particularly important to do this now because researchers are planning to send life-detection instruments to destinations such as the solar system moons Europa, Enceladus and Titan, more exotic than most of the worlds visited so far. “Most of these other places we’re beginning to think about as targets for astrobiology are really weird and different,” Johnson says. If you’re going to a weird and different place, you might expect weird and different life, squirming invisibly beyond the reach of a lamppost’s light.
Their pitch worked: The expanded lunch table became LAB. Now the project, a spread-out coalition of scientists more than a single physical laboratory, is a few years deep into its work. The researchers aim to learn how things like the complexity of a surface, anomalous concentrations of elements and energy transfer—such as the movement of electrons between atoms—might reveal life as no one knows it.
LAB Work
LAB’s research is a combination of fieldwork, lab projects and computation. One project is a planned visit to Canada’s Kidd Creek Mine, which drops nearly 10,000 feet into the ground. Its open pit looks like a quarry reaching toward the seventh circle of hell. At those depths, around 2.7 billion years ago, an ocean floor brewed with volcanic activity, which left sulfide ore behind. The conditions are similar(ish) to what astronomers believe they might find on an “ocean world” like Europa. In the mine, the scientists hope to probe the differences between minerals that formed by crystallization—when atoms fall out of solution and into an ordered, lattice structure in the same place they are now—and evidence of biology.
The two kinds of materials can look superficially alike because they’re both highly ordered. But the team aims to show that geochemical models, which simulate how water saturated with chemicals will precipitate them out, will predict the kind of abiotic crystals found there. Kidd Creek, for instance, has its own sort: Kiddcreekite, a combination of the copper, tin, tungsten and sulfur that crystallizes from the water. Those same models, however, aren’t likely to predict biological structures, which form according to different forces and rules. If that turns out to be true, the models may prove useful when applied to alien geochemical conditions to predict the naturally forming minerals. Anything else that’s found there, the thinking goes, might be alive.
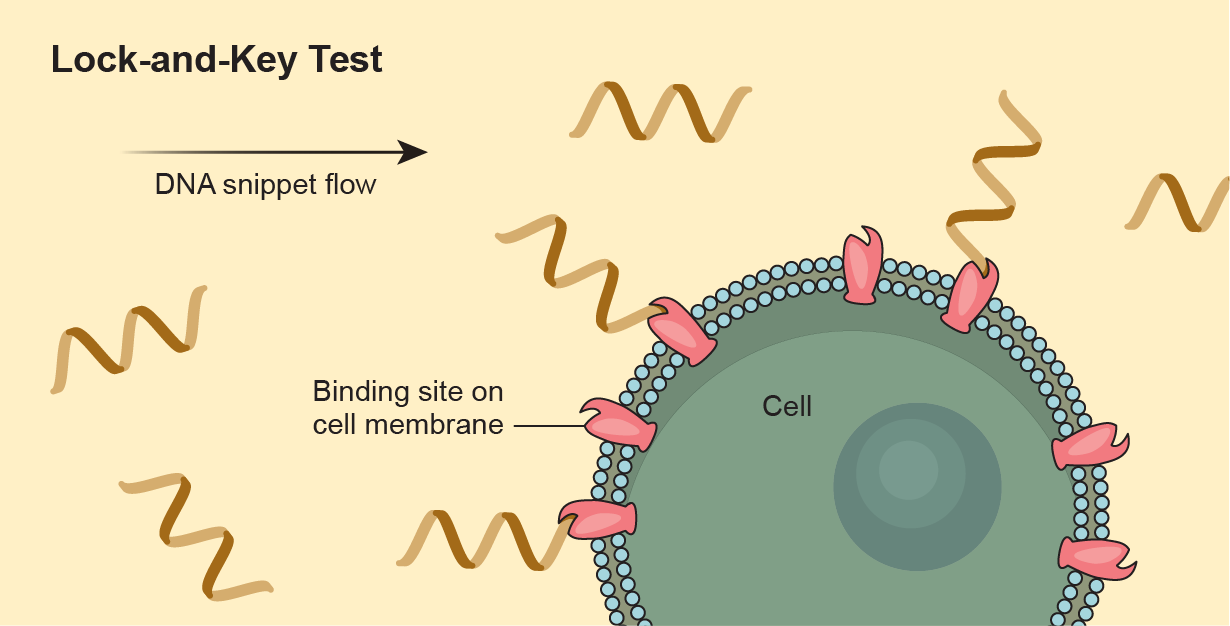
Johnson is reaching back to her postdoc days, using the genetic sequencers whose relevance she called into question back then. The group, though, has found a way to make them more agnostic. The researchers plan to use the instruments to investigate the number of spots on a cell’s surface where molecules can attach themselves—like the places where antibodies stick to cells. “We had this hypothesis that there are more binding sites on something complicated like a cell than a small particle,” Johnson says, such as an unalive mote of dust. Something alive, in other words, should have more lock-and-key places.
To test this idea, they create a random pool of DNA snippets and send it toward a cell. Some snippets will hook up with the cell’s exterior. The scientists next remove and collect the bound snippets, then capture the unbound snippets and send them back to the target cell again, repeating the process for several cycles. Then they see what’s left at the end—how much has hooked on and how much is still free. In this way, the researchers can compare the keys locked into the cell with those attached to something like a dust particle.
The scientists will also scrutinize another key difference they suspect divides life and not-life: Things that are not alive tend to be at a kind of equilibrium with their environment. In contrast, something that’s alive will harness energy to maintain a difference from its surroundings, LAB member Peter Girguis of Harvard hypothesizes. “It’s using power to keep ourselves literally separate from the environment, defining our boundary,” he says. Take this example: When a branch is part of a tree, it’s alive, and it’s different—in a bordered way—from its environment. If you remove that life from its energy source—pluck the branch—it dies and stops using power. “In a matter of time, it disintegrates and becomes indistinguishable from the environment,” Girguis says. “In other words, it literally goes to equilibrium.”
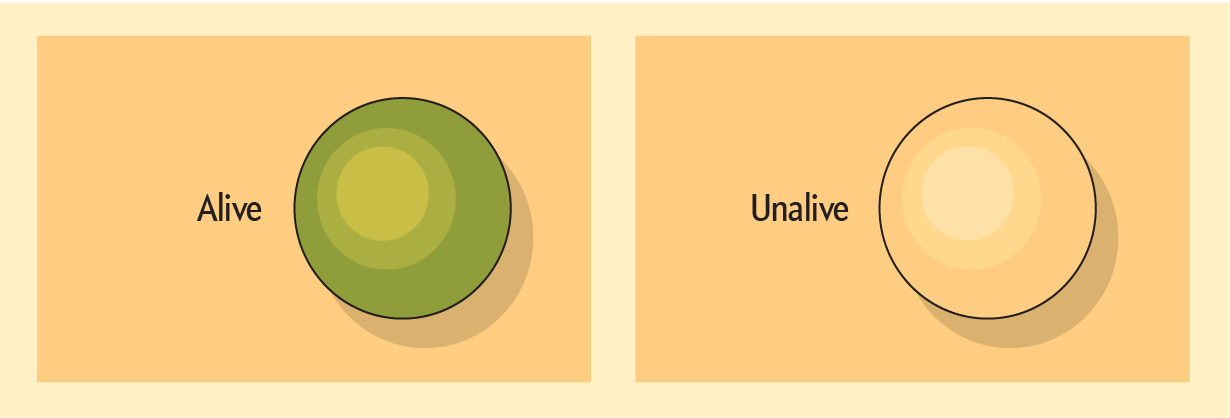
The disequilibrium of living should show up as a chemical difference between an organism and its surroundings—regardless of what the surroundings, or the life, are made of. “I can go scan something, make a map and say, ‘Show me the distribution of potassium,’” Girguis says. If blobs of concentrated K appear, dotting the cartography only in certain spots, you may have biology on your hands.
Girguis’s LAB work intertwines with another pillar of the group’s research: a concept called chemical fractionation, which is how life preferentially uses some elements and isotopes and ignores others. A subgroup investigating this idea, led by Christopher House of Pennsylvania State University, can use the usual data that space instruments take to suss out the makeup of a planet or moon. “If you understand the fundamental rules about the inclusion or exclusion of elements and isotopes, then you can imagine a different ecosystem where it still behaves by similar rules, but the elements and isotopes are totally different,” House says. It could give disequilibrium researchers a starting point for which kinds of patterns to focus on when making their dotted maps.
Within House’s group, postdoc researchers are studying sediments left by ancient organisms in Western Australia. Looking at these rock samples, they try to capture patterns showing which elements or isotopes early Earth life was picky about. “We’re hopeful that we can start to generalize,” House says.
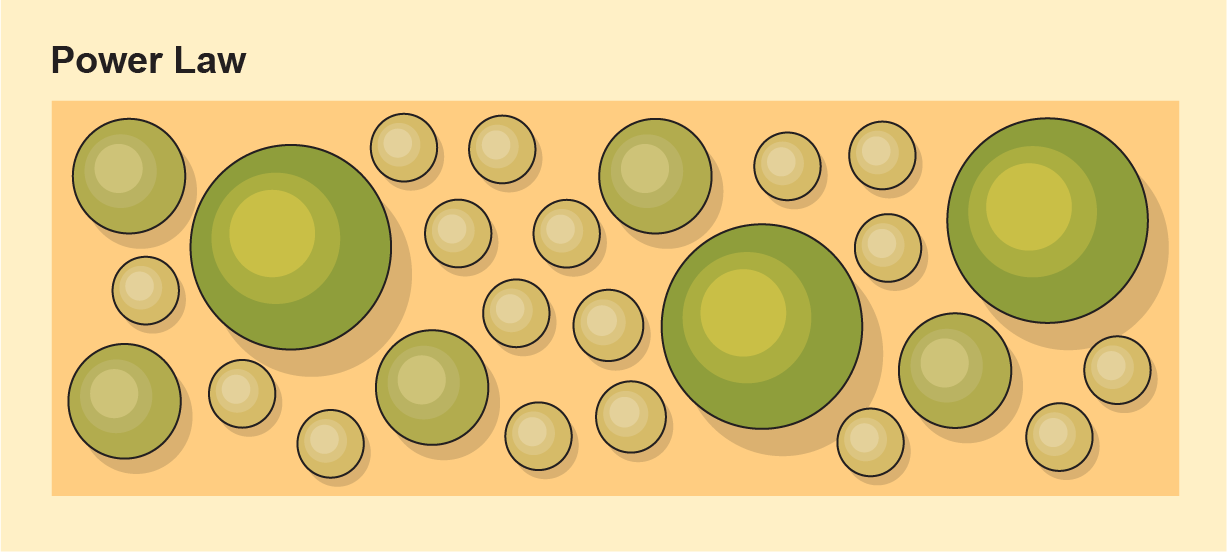
LAB’s computing team, co-led by Chris Kempes of the Santa Fe Institute, is all about such generalizing. Kempes’s research focuses on a concept called scaling—in this case, how the chemistry inside a cell changes predictably with its size and how the abundance of different-sized cells follows a particular pattern. With LAB, Kempes, House, Graham and their collaborators published a paper in 2021 in the Bulletin of Mathematical Biology about how scaling laws would apply to bacteria. For instance, if you sort a sample of biological material by size, differences pop out. Small cells’ chemistry looks a lot like their environment’s. “The bigger cells will be more and more different from the environment,” Kempes says.
The abundance of cells of different sizes tends to follow a relationship known as a power law: Lots of small things with a steep drop-off as cells get larger. If you took an extraterrestrial sample, then, and saw those mathematical relationships play out—small things that looked like their surroundings, with progressively larger things looking less like their environments, with lots of the former and few of the latter—that might indicate a biological system. And you wouldn’t need to know ahead of time what either “environment” or “biology” looked like chemically.
Cronin, a sort of heretic within this heretic group, has his own idea for differentiating between living and not. He’s an originator of something called assembly theory, a “way of identifying if something is complex without knowing anything about its origin,” he says. The more complex a molecule is, the more likely it is to have come from a living process.
That can sound like a bias in the agnosticism, but everyone generally concedes that life results from, as Sutherland puts it, “the complexification of matter.” In the beginning, there was the big bang. Hydrogen, the simplest element, formed. Then came helium. Much later there were organic molecules—conglomerations of carbon atoms with other elements attached. Those organic molecules eventually came together to form a self-sustaining, self-replicating system. Eventually that system started to build the biological equivalent of 747s (and then actual 747s).
In assembly theory, the complexity of molecules can be quantified by their “molecular assembly number.” It’s just an integer indicating how many building blocks are required to bond together, and in what quantities, to make a molecule. The group uses the word “abracadabra” (magic!) as an example. To make that magic, you first need to add an a and a b. To that ab, you can add r. To abr, toss in another a to make abra. Then attach a c, then an a and then a d, and you get abracad. And to abracad, you can add the abra that you’ve already made. That’s seven steps to make abracadabra, whose molecular assembly number is thus seven. The group postulated that a higher number meant a molecule would have a more complicated “fingerprint” on a mass spectrometer—a tool that separates a sample’s components by their mass and charge to identify what it’s made of. A complex molecule would show more distinct peaks of energy, in part because it was made of many bonds. And those peaks are a rough proxy for its assembly number.
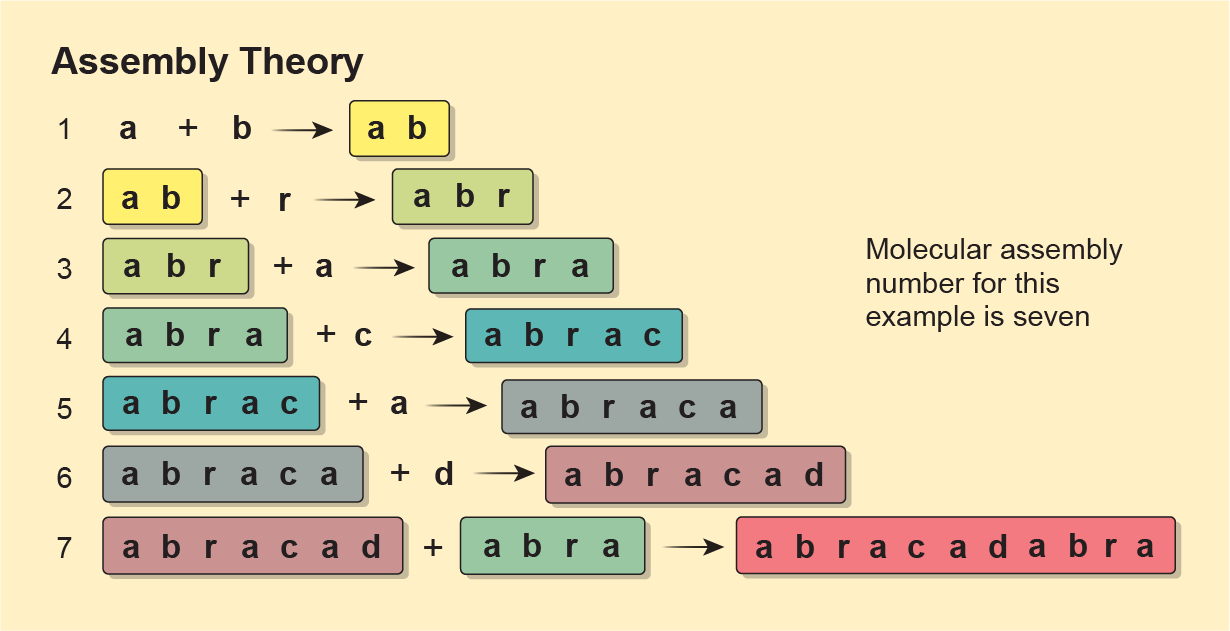
Cronin had bragged that by doing mass spectrometry, he could measure the complexity of a molecule without even knowing what the molecule was. If the technique indicated that a molecule’s complexity crossed a given threshold, it probably came from a biological process.
Still, he needed to prove it. Through LAB, NASA gave him double-blind samples of material to yea or nay as biological. The material hailed from outer space, fossil beds and the sediments of bays, among other places. One of the samples was from the Murchison meteorite, a 220-pound hunk of rock, full of organic compounds. “They thought the technique would fail because Murchison is probably one of the most complex interstellar materials,” he says. But it succeeded: “It basically says Murchison seems a bit weird, but it’s dead.”
Another sample contained 14-million-year-old fossils, sculpted by biology but meant to fool the method into a “dead” hit because of their age. “The technique found that they were of living origin pretty easily,” Cronin says. His results appeared in Nature Communications in 2021 and helped to convince Cronin’s colleagues that his line of research was worthy. “There are a lot of skeptical people in [LAB’s] team, actually,” he says.
Aliens Discovered??
There is plenty of skepticism outside LAB as well. Some scientists question the need to search for unfamiliar life when we still haven’t done much searching for extraterrestrial life as we know it. “I think there’s still a lot we can explore before we go to life as we don’t know it,” says Martina Preiner of the Royal Netherlands Institute for Sea Research and Utrecht University.
Still, even among old-school astrobiology researchers looking for Earth-like signatures on exoplanets, the LAB approach has support. Victoria Meadows of the University of Washington has been thinking about such far-off signals for two decades. She’s seen the field change over that time—complexify, if you will. Scientists have gone from thinking “if you see oxygen on a planet, slam dunk,” to thinking “there are no slam dunks.” “I think what my team has helped provide and how the field has evolved is this understanding that biosignatures must be interpreted in the context of their environment,” she says. You have to understand a planet’s conditions, and those of its star, well enough to figure out what oxygen might mean. “It may be that the environment itself can either back up your idea that oxygen is due to life or potentially that the environment itself may produce a false positive,” she says, such as from an ocean boiling off.
In a lot of ways, Meadows says, looking for agnostic biosignatures is the ultimate way to take such cosmic conditions into account. “You have to understand the environment exquisitely to be able to tell that something anomalous—something that isn’t a planetary process—is operating in that environment,” she says. Still, this variety of alien hunting is in its infancy. “I think they’re really just starting off,” she says. “I think what LAB is doing in particular is a pioneering effort on really getting some science under this concept.”
Even so, Meadows isn’t sure how likely LAWDKI is. “The question is, ‘Is the environment on a [terrestrial] extrasolar planet going to be so different that the solutions are so different?’’ Meadows asks. If the conditions are similar and the chemicals are similar, it’s reasonable to think life itself will be similar. “We are expecting to see some similar science if these environments are similar, but of course I will expect that there’ll be things that will surprise us as well.” It’s for all these reasons that Meadows, whose work focuses on exoplanets, is working with the LAB scientists, whose research for now homes in on the solar system, to bring their two worlds together.
By the end of LAB’s grant, the team plans to develop instruments that will help spacecraft notice weird and different life close to home. “We’re extremely focused on the ultimate goal—how we can take these tools and techniques and help develop them to the point they can become instruments on space missions,” Johnson says.
No one piece of information, gathered from a single instrument, can reliably label something life, though. So the group is working toward suites of devices, drawing on all their focus areas, that work together in different environments, such as worlds wrapped in liquid versus rocky deserts. Graham is gathering sample sets that LAB’s subgroups can test in a round-robin way to see how the superimposition of their results stacks up. They might look for, say, molecules with big assembly numbers concentrated in bounded areas that look different from their environment.
Even if these approaches collectively find something, it’s unlikely to provide a definitive answer to the question “Are we alone?” It will probably yield a “maybe,” at least for a while. That grayness may disappoint those who’d like “Aliens discovered!” headlines, instead of “Aliens discovered?? Check back in 10 years.”
“I understand that frustration,” Johnson says, “because I’m a restless sort of person.” That restlessness relates in part to her own mortality. The end of the time when she’s out of equilibrium with her environment. The demise of her complexity, of her detectability and ability to detect. “We have these ephemeral lives,” she says. “We have this world that’s going to end. We have this star that’s going to die. We have this incredible moment. Here we are: alive and sentient beings on this planet.” All because, at some point, life started.
That may have happened tens or hundreds or thousands or millions or billions of other times on other planets. Or, maybe, it has only happened here. “It just feels,” Johnson says, “like an extraordinary thing that I want to know about the universe before I die.”
This article was originally published with the title “Life as We Don’t Know It” in Scientific American 328, 2, 32-39 (February 2023)
doi:10.1038/scientificamerican0223-32
From our Archives
The Search for Extraterrestrial Life. Carl Sagan; October 1994.

